Research

The Mechanism(s) for the Photocatalystic Reduction of Carbon Dioxide
Though the time scale for the depletion of fossil fuel is debatable there is no doubt that we will eventually run out of fossil fuels as the major fuel source for the world. As such, it is prudent for science to begin to focus on improving methods for the production of fuels from renewable sources.
Our work is currently centered on two projects that are focused on understanding how transportation fuels can be produced from renewable energy sources. One project focuses on the photocatalytic reduction of CO2. This is in many ways the “holy grail” of renewable energy research. It will be a monumental achievment if it proves possible to use solar energy to efficiently photocatalytically reduce CO2 to fuels using a renewable energy source in a process that would be carbon neutral. In addition to providing a renewable energy source, this process would not have a negative impact on the carbon dioxide balance in the atmosphere. Of course, the issue here is not whether this is possible --- the process is possible. The photoreduction of carbon dioxide has been reported for more than 30 years. The issue is one of efficiency. Currently the yields of hydrocarbons produced via photoreduction of carbon dioxide are exceedingly low. Until yields are increased significantly, the cost of fuels produced by such a process will remain unrealistically high and the process will not be economically viable.
Our approach is to develop an understanding of the mechanism(s) involved in the photoreduction of carbon dioxide. Our studies are focused on elucidating the microscopic molecular level reactions involved in the photoreduction of carbon dioxide. The overall process for the transformation of CO2 to methane can be written as:
CO2+ 4H2 → CH4 + 2H2O. (1)
The equation that is written above involves a possible stoichiometric reaction for the conversion of carbon dioxide to methane, but what is written in (1) is certainly not an elementary reaction. Given that hydrogen must be supplied to form methane, and that the reaction would normally be envisioned to take place in an environment in which water is present, the above reaction is often written in the following form:
CO2 + 2H+ + 2e- → HCOOH
HCOOH + 2H+ + 2e- → HCHO + H2O
HCHO + 2H+ + 2e- → CH3OH
CH3OH + 2H+ + 2e- → CH4 + H2O
Though this reaction scheme looks plausible there is no clear evidence that this is indeed the mechanism for the overall reaction in (1). In fact, there is no evidence that any of the reactions listed above are elementary reactions, and other plausible reactions schemes can be written. For example, there has been considerable discussion in the literature as to the role of CO2-, the anion of carbon dioxide, in the photocatalytic conversion of CO2. There is intrinsic appeal to the involvement of such a species, since the above set of equations make it clear that independent of the mechanism that is actually operative 8 electrons will be needed to transform carbon dioxide to methane. Even if other fuels, such as methanol or formic acid, were targets, they too would by necessity involve multi-electron processes. How multiple electrons, which are generated on irradiation of a semi-conductor catalyst, are tranported to and tranfer to the appropriate reactants adsorbed at desired reaction sites, is one of the puzzles and challenges of this research. CO2- has appeal as a reaction intermediate in that it can be envisioned as being produced by a one electron transfer to carbon dioxide and thus it would act as a starting point for the rest of the reaction scheme.
Our goal is to delineate the molecular level reaction steps that take place in the mechanism for the photoreduction of carbon dioxide. Of course, these steps may, and probably do, vary as a function of catalyst and possibly reaction environment. Probing the effect of reaction conditions on the overall mechanism is another important part of our research program.
Clearly this is a complex problem and, as such, we are approaching this problem with a collaborative approach under the sponsorship of the Institute for Catalysis in Energy Processes (ICEP) - http://www.iec.northwestern.edu . We are collaborating with other researchers in the ICEP and at Argonne National Laboratory who have expertise in cutting edge theoretical work and in various types of modern spectroscopies. The theory work is providing us with insights into which types of reactions and reaction mechanisms are energetically and kinetically plausible for the desired transformations. The results from other types of spectroscopic studies, including EPR and Raman, are and will be used in conjunction with our results, which are primarily based on IR and time resolved IR studies of relevant reactions on surfaces, to construct a very detailed and comprehensive picture of the overall reaction process.
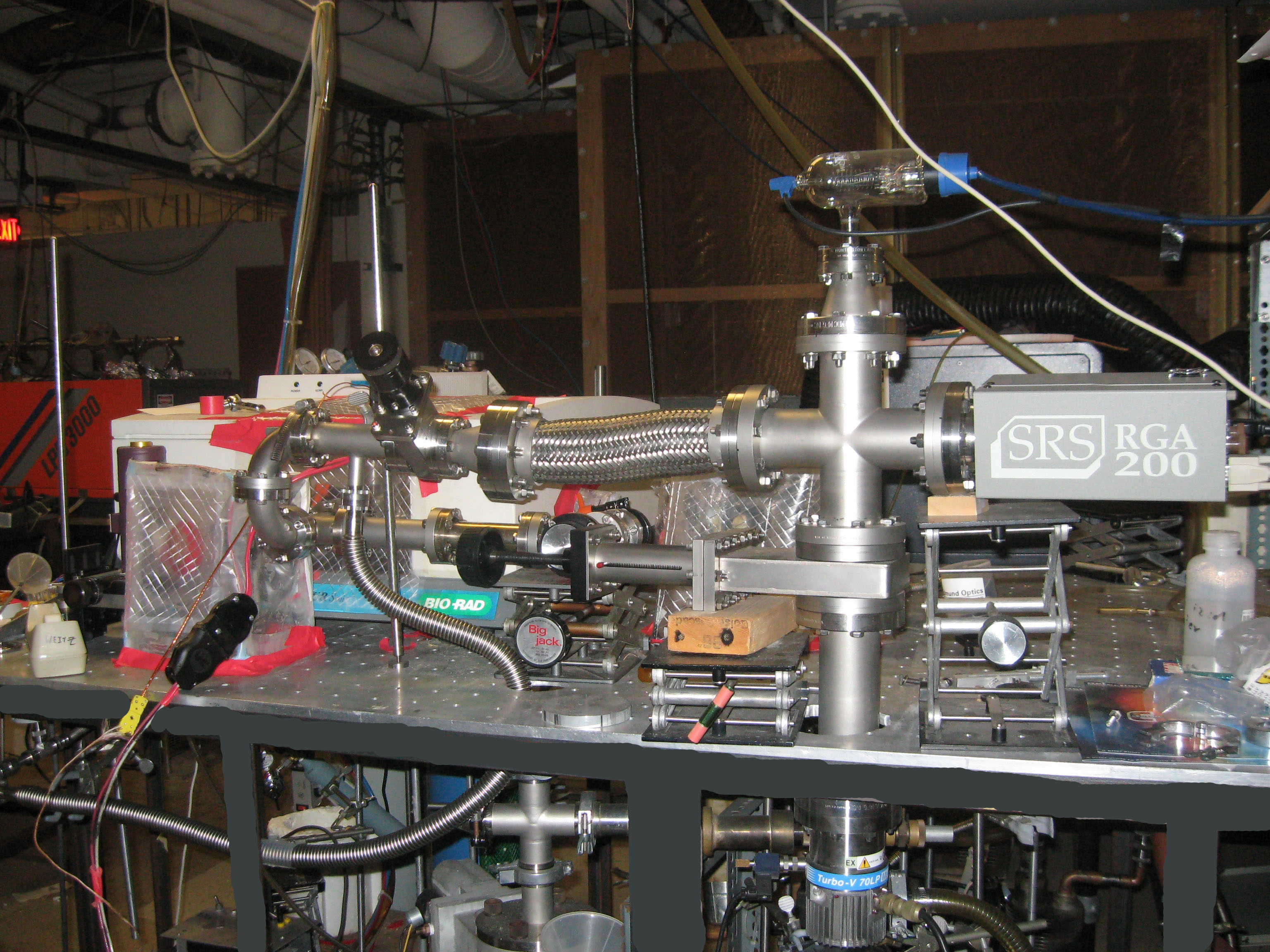
We are focusing on both the photocatalytic and the electrocatalytic reduction of carbon dioxide. The latter is in collaboration with Professor R.P. Van Duyne. In the former area we make use of a technique that we have used very successfully in the elucidation of the catalytic reaction mechanism for NOx removal from diesel exhaust. This was also a very complex problem that had real world implications and applications. This technique employs what we call a wire grid reactor. This reactor allows us to support highly scattering powders, such as photocatalytic metal oxides, on a highly transmissive tungsten grid and study reactions that take place using infrared and/or time resolved IR in a transmission mode. We find that with transmission IR, as opposed to diffuse reflectance IR (DRIFT) we can more readily compare signal intensities from one experiment to another – a feature that significantly facilitates mechanistic investigations. A photo of this apparatus is shown below with a quadrupole mass spec coupled to the vacuum system.
This apparatus can be operated in a time resolved mode. However, we also have laser based irradiation and probe sources that can be employed for shorter timescale time resolved IR studies. Another component of this project is the synthesis of new catalytic materials using both facilities available in our laboratories and in collaboration with others in the ICEP. One of our synthetic vaccum lines is shown below.
We are optimistic that this approach will yield new and important insights into the mechanism for photoreduction of carbon dioxide and we hope that these insights will lead to improvements in the efficiency and yield for this process.
The Mechanism(s) for the Breakdown of Fructose to Simpler Compounds
Another renewable source of fuels is biomass. We are focusing on gaining an understanding of the reaction pathways in the degradation of sugars, including sucrose, glucose and fructose to chemicals that can be used as, or used to produce, liquid fuels. This work is sponsored by the Institute for Atom Efficient Chemical Transformations (IACT) which is a DOE funded EFRC (http://www.anl.gov/catalysis-science/). Other researchers within the Institute are working on the custom design of catalytic materials. Our concept for the custum design of catalysts is revolutionary in that we are developing methods to assemble materials with multiple reactive sites where the nature and proximity of these sites can be controlled at an atom level. Thus, once a reaction mechanism is understood and the step(s) that lead to desired transformation are identified, we believe it will be possible to design catalysts that enhance these desirable pathways and improve both the efficiency and selectivity of desirable processes. There is also state of the art computational work being done in IACT, both to help identify productive research directions for experimentalists and to help in the understanding of experimental results.
We are currently studying the mechanism for reaction of fructose to produce levulenic acid and formic acid. Formic acid can be used directly as a “fuel” in a fuel cell and levulenic acid can be readily converted to liquid transportation fuels. As shown below, this reaction is thought to proceed through HMF. However, there is little experimental data that provides insights into the details of this reaction at a molecular level.
We are currently using 13C NMR to probe the reactions of fructose labeled with 13C at different positions to determine the position at which the 13C label ends up in HMF and in levulenic acid and/or formic acid. We expect that these data will allow us to infer information on reaction intermediates and reaction pathways. We are also using 13C NMR and ATR FTIR techniques to directly probe reaction intermediates. We also anticipate using in situ Raman in these studies.